What Happens to Snowflakes as They Become Progressively Buried Within Glaciers?
Abstract
Nosotros analyzed the dielectric spectra (0.1 Hz–1 MHz) of 49 firn and ice samples from ice sheets and glaciers to better understand how differing ice formation and development impact electrical properties. The dielectric relaxation of ice is well known and its characteristic frequency increases with the concentration of soluble impurities in the ice lattice. Nosotros found that meteoric water ice and firn generally possess 2 such relaxations, indicating singled-out crystal populations or zonation. Typically, one population is consistent with that of relatively pure water ice, and the other is significantly more impure. All the same, high temperatures (eastward.g. temperate ice), long residence times (e.g. ancient ice from Mullins Glacier, Antarctica) or anomalously high impurity concentrations favor the development of a unmarried relaxation. These relationships suggest that annealing causes two dielectrically distinct populations to merge into one population. The dielectric response of temperate water ice samples indicates increasing purity with increasing depth, suggesting ongoing rejection of impurities from the lattice. Separately, subglacially (lake) frozen samples from the Vostok (Antarctica) 5G ice core possess a single relaxation whose variable characteristic frequency likely reflects the composition of the source water. Nosotros conclude that multi-frequency methods are essential to dielectric discrimination between unlike types of glacier ice.
Introduction
The electrical properties of glacier water ice have been investigated over many orders of magnitude in frequency to help interpret its concrete chemistry (due east.yard. Reference Wolff, Miners, Moore and ParenWolff and others, 1997; Reference Stillman, MacGregor and GrimmStillman and others, 2013a), density and material (eastward.m. Reference Fujita, Matsuoka, Ishida, Matsuoka, Mae and HondohFujita and others, 2000), the nature of polar ice sheets (e.g. Reference Dowdeswell and EvansDowdeswell and Evans, 2004; Reference Eisen, Wilhelms, Steinhage and SchwanderEisen and others, 2006; Reference MacGregorMacGregor and others, 2015) and paleoclimate (due east.g. Reference TaylorTaylor and others, 1993; Reference Wolff and HondohWolff, 2000; WAIS Divide Project Members, 2013). Water ice cores are analyzed electrically using several logging methods, most normally the near-d.c. electrical conductivity measurement (ECM; Reference HammerHammer, 1980) and loftier-frequency (up to 300 kHz) dielectric profiling (DEP; Reference Moore and ParenMoore and Paren, 1987; Reference Wilhelms, Kipfstuhl, Miller, Heinloth and FirestoneWilhelms and others, 1998). These tools are optimized for rapid, continuous logging of ice cores. Nonetheless, a broadband spectrum is required to characterize dielectric dispersion in a fabric (e.g. Reference Barsukov and MacdonaldBarsukov and Macdonald, 2005). Specifically, measurements distributed from i Hz to 100 kHz are necessary to resolve the dielectric relaxations present in ice, and to divide them from d.c. conductivity. Although some progress has been made in multi-frequency core profiling (e.m. Reference Sugiyama and HondohSugiyama and others, 2000), just laboratory measurements of private ice samples take provided the dense frequency sampling necessary for robust characterization of the dielectric properties of glacier ice (due east.g. Reference Fitzgerald and ParenFitzgerald and Paren, 1975; Reference MaenoMaeno, 1978; Reference Stillman, MacGregor and GrimmStillman and others, 2013a). In contrast, measurements ≫100 kHz respond only to the sum of d.c. and dielectric conductivity.
This study is motivated by new broadband measurements of polar and temperate glaciers, reported herein, that show both similarities to and differences with ice-sheet spectra. These relationships propose general features of dielectric spectra that rails the formation and evolution of all glacier water ice. We first review the fundamentals of dielectric spectroscopy and the salient properties of earlier observations equally they relate to diagnostic dielectric signatures of dissimilar types of glacier ice, setting aside detailed implications for physical chemistry that we have considered previously (Reference Grimm, Stillman, December and BullockGrimm and others, 2008; Reference Stillman, Grimm and DecemberStillman and others, 2010, Reference Stillman, MacGregor and Grimm2013a,Reference Stillman, MacGregor and Grimmb). We distinguish here between meteoric and accreted ice in polar ice sheets and polar and temperate glaciers, and we differentiate firn from ice for all sources. We evaluate these backdrop in terms of the formation and evolution of ice masses. We conclude by discussing the value of these dielectric signatures for interpreting several types of measurements, including dielectric spectroscopy of individual water ice-core samples, electrical logs of full cores, and geoelectrical measurements in boreholes and soundings from the surface.
Background
Materials respond to time-varying electric fields by a combination of storage and dissipation of energy, which tin exist represented by a unmarried, frequency-dependent complex number. Hither nosotros focus on circuitous permittivity ![]() . Other formulations are possible (e.grand. complex resistivity or conductivity). The existent permittivity ε′ determines energy storage (capacitance), and the imaginary permittivity ε″ determines energy dissipation (resistance). In principle, one can be predicted from the other using the Kramers–Kronig relations and assuming space bandwidth, but, in practice, measuring both gives greater robustness to fitting of bandwidth-limited spectra with multiple constituents.
. Other formulations are possible (e.grand. complex resistivity or conductivity). The existent permittivity ε′ determines energy storage (capacitance), and the imaginary permittivity ε″ determines energy dissipation (resistance). In principle, one can be predicted from the other using the Kramers–Kronig relations and assuming space bandwidth, but, in practice, measuring both gives greater robustness to fitting of bandwidth-limited spectra with multiple constituents.
Polarizability arises from specific charges, mobilities and length scales. It ofttimes follows a dispersion called a dielectric relaxation that is due to the frequency-dependent ability of charges to motion within an applied electric field. The relaxation frequency f r is the frequency at which charges motion over most their maximum possible extent during a single applied cycle. A useful description of a dielectric relaxation is the Cole–Cole model (Reference Cole and ColeCole and Cole, 1941). In general, we fit dielectric spectra to a sum of Cole–Cole relaxations plus a static (d.c.) electrical conductivity:
(1)
![]()
where Δεi , τi and αi are the dielectric susceptibility, relaxation time (1/iiπf r) and Cole–Cole distribution parameter, respectively, for the ith dielectric relaxation, ω is the angular frequency, ε ∞ is the high-frequency-limit permittivity, σ DC is the d.c. conductivity and ε 0 is the permittivity of the vacuum. The parameter α increases with the broadening of the distribution of length scales of charge motion. For a Debye relaxation (Reference DebyeDebye, 1929), with a single such length scale, α = 0. Dielectric relaxations are distinguished by an inflection in ε′ and a peak in ε″. The distinct appearance of the latter feature simplifies identification and Cole–Cole plumbing equipment.
The real office of the electrical conductivity will also approach a loftier-frequency asymptote σ ∞ for a Debye relaxation (at any frequency, σ = εω), simply in the general example of α > 0 there is a continued positive slope. For this reason, we study high-frequency electrical conductivity σ HF at 300 kHz when discussing this quantity.
Between −forty°C and −xx°C, ice relaxation frequencies are typically >100 Hz and <one kHz, then this range in f r can lead to meaning dispersion betwixt ∼i Hz and ∼100 kHz. At radio frequencies (>1 MHz), the asymptotic electrical conductivity due to ice relaxation tin be inferred, but the ice dispersion itself is non resolved. Furthermore, the close agreement of the −20°C dielectric constant at 1 MHz (three.20 ± 0.02: Reference Fujita, Matsuoka, Ishida, Matsuoka, Mae and HondohFujita and others, 2000) and the dielectric abiding at 10 MHz–1.5 GHz (3.18 ± 0.01: Reference Bohleber, Wagner and EisenBohleber and others, 2012) implies that dispersion is minimal and therefore there are no pregnant undiscovered dielectric relaxations of whatever kind beyond this ring.
Both polarizability and conductivity in ice are due to protonic charge defects: lattice sites where hydrogen atoms are configured improperly with respect to next H2O molecules (Reference Petrenko and WhitworthPetrenko and Whitworth, 1999). Ice contains a stock-still density of intrinsic protonic defects, and extrinsic defects are generated by sure ions (impurities) that are soluble in the water ice lattice (e.g. Reference Camplin, Glen and ParenCamplin and others, 1978). In do, even the purest laboratory-grown ice contains a small proportion of extrinsic defects that are electrically detectable (Reference KawadaKawada, 1978). Dielectric relaxations are generally shut to but distinct from the ideal Debye form. Reference MacGregorMacGregor and others (2015) demonstrated that the effect of non-Debye behavior on conductivity was critical to understanding the relationship between radar attenuation and temperature inside the Greenland ice sheet.
For meteoric water ice, the dominant soluble impurities are H+ from volcanic acids, Cl− from body of water table salt and NHfour + from biomass called-for (Reference Wolff, Miners, Moore and ParenWolff and others, 1997). Reference JaccardJaccard (1959) described the relationships between defect and electrical properties in single-crystal ice, which we refer to time to come as Jaccard theory (see Appendix). Reference Stillman, MacGregor and GrimmStillman and others (2013a) showed that the complex permittivity from d.c. to 1 MHz of meteoric polar ice can be fully explained by lattice-soluble impurities using Jaccard theory. Grain boundaries human activity every bit resistors that impede d.c. conduction, instead of providing interconnected conductive pathways as previously hypothesized (Reference Wolff and ParenWolff and Paren, 1984; Reference KulessaKulessa, 2007). Reference Stillman, MacGregor and GrimmStillman and others (2013a) likewise plant that in situ 'd.c.' geoelectrical soundings of water ice sheets actually amend match laboratory-measured trends in σ ∞, therefore those before field measurements are unreliable. The true bulk d.c. conductivity is much lower than those field data, which invalidates before models explaining differences between temperate and polar ice that were based on perceived central differences in their d.c. conductivities (Reference Gross, Wong and HumesGlen and others, 1977; Reference Reynolds and ParenReynolds and Paren, 1980).
The lattice impurity concentration in polycrystalline ice depends not but on the available sources, merely also on the mode of formation. The partition coefficient k from liquid to the water ice lattice is small for slowly frozen laboratory water ice (∼10−three; Reference Gross, Wong and HumesGross and others, 1977; Reference Grimm, Stillman, Dec and BullockGrimm and others, 2008), because ice formed in this way very efficiently ejects impurities to the grain boundaries. In contrast, yard = 0.3–0.8 for meteoric ice-sheet samples (Reference Stillman, MacGregor and GrimmStillman and others, 2013a). These high sectionalization coefficients were derived by comparing Jaccard theory to an empirical relationship between σ HF and bulk impurity concentrations (Reference Wolff, Miners, Moore and ParenWolff and others, 1997; Appendix). Therefore impurity numbers in ice sheets are comparable between the lattice (where low volumetric concentrations are difficult to observe directly) and the much smaller grain boundaries (where higher concentrations tin can be detected using microscopic imaging (e.yard. Reference Mulvaney, Wolff and OatesMulvaney and others, 1988)). We hypothesize that this behavior results from faster nucleation compared to laboratory freezing and consistent incomplete impurity ejection (Reference Stillman, MacGregor and GrimmStillman and others, 2013a). This difference in g explains why Reference Fitzgerald and ParenFitzgerald and Paren (1975) could non reproduce the electrical properties of a glacier ice sample that they melted and refroze. Weak partitioning from liquid also explains why water ice in permafrost is nigh electrically pure (Reference Grimm and StillmanGrimm and Stillman, 2015), i.east. it has few extrinsic defects.
Samples and methods
Water ice-core samples
We include all 26 Antarctic and Greenland samples measured by Reference Stillman, MacGregor and GrimmStillman and others (2013a), who focused on conduction mechanisms in meteoric polar ice. Nosotros include new measurements from the Vostok (Antarctica) 5G ice core that include both types of accreted ice constitute within that core (Reference JouzelJouzel and others, 1999; Reference De Angelis, Petit, Savarino, Souchez and ThiemensDe Angelis and others, 2004). We supplement these ice-sheet samples with two laboratory dielectric spectra from the earlier literature: the first is a deep sample from the 1968 Byrd Station (Antarctica) water ice core (Reference Paren and GlenParen and Glen, 1978) and the second is a firn sample taken from the Palmer Land plateau, Antarctic Peninsula (Reference ReynoldsReynolds, 1985). We complete our survey of glacier water ice with samples from two tall glaciers: Upper Fremont Glacier, Wyoming, U.s.a., and Mullins Glacier, Beacon Valley, Antarctica. Upper Fremont Glacier is a typical temperate mountain glacier whose high acme and accumulation rate accept proven useful for paleoclimate studies of the past several centuries (Reference Schuster, White, Naftz and CecilSchuster and others, 2000). Mullins Glacier is a cold-based, debris-covered glacier ∼8 km long; isotopic measurements of the overlying till suggest a maximum historic period ∼8 Ma (Reference MarchantMarchant and others, 2007). The terminus of this glacier, and a buried relict of Taylor Glacier that abuts it (Reference SugdenSugden and others, 1995), may be the oldest water ice on Earth. Table 1 summarizes the origins, depths, ages and visual characteristics of the ice samples included in this analysis. All samples were obtained from the US National Ice Core Laboratory and were cutting at that place to thicknesses of 7–14 mm. Samples were stored in our laboratory in a humidified freezer at −40°C for a period up to several months earlier measurement.
Tabular array 1. Key characteristics of ice samples evaluated in this study

Dielectric measurements
Samples were measured between two circular electrodes, an upper 55 mm diameter unguarded electrode and a lower guarded electrode whose diameter (x–30 mm) depended on the sample bore (Solartron 12963A sample holder). The grounded guard (3rd) electrode was sized to match the diameter of the composite lower electrode to the upper electrode. Complex impedance was measured as a office of frequency and temperature using a Solartron 1260A Impedance Analyzer with a 1296A Dielectric Interface. This system is capable of measuring conductivities equally low as ten−12 Due south g−ane and phases of 0.1 mrad. The impedance was converted to complex permittivity using the electrode geometry. Uncertainties estimated from repeat measurements are typically 5% and ten% for ε′ and ε″, respectively. For Mullins Glacier samples, some of which could not be microtomed due to englacial debris, there was no difference in dielectric signature between articulate samples that were microtomed, and discolored or dirty samples that were not and that were frozen onto warmed electrodes instead.
Measurements were swept from high to depression frequency, from 10half-dozen to ten−1 Hz (nosotros rarely exploit the arrangement's lower limit of 10−3 Hz due to measurement time). Because measurements are more reproducible during warming and eliminate the possible confounding effects of meta-stable water, we typically cool samples slowly to −90°C and and so collect data during slow warming (0.1°C min−1), with more 30 min stabilization at each 2.5°C measurement interval. Nosotros observed no discontinuities indicating stage transitions. The big temperature range allows frequency-overlapping polarization mechanisms to be identified past the temperature dependence (activation energy) of their individual relaxation frequencies.
Modeling of dielectric spectra
At each temperature, the complex permittivity spectra were modeled as a sum of Cole–Cole relaxations and d.c. conductivity (Eqn (1)). We annotation that even if a spectral gradient remains at low frequency, the d.c. conductivity tin still be recovered accurately past this modeling procedure. We fit both ε′ and ε″ simultaneously; the model root-mean-square (RMS) misfit for both quantities was typically ∼i%. 95% confidence premises on the Cole–Cole parameters for each relaxation were estimated using changes in goodness of fit (Δχ two distributions). The feature frequency for each relaxation reflects the full number of defects according to Jaccard theory (see Appendix). Reference Grimm, Stillman, Dec and BullockGrimm and others (2008) and Stillman and others (Reference Stillman, Grimm and Dec2010, Reference Stillman, MacGregor and Grimm2013a) provide additional details of measurement and plumbing equipment procedures.
Results
Effigy ane shows the dielectric spectra of all samples at approximately −40°C, illustrating the range of dielectric behaviors observed in glacier ice. This temperature was the highest measured across all samples (some were measured at higher temperatures, merely not uniformly then) and was selected to avoid whatever potential pre-melting furnishings. Even so, we note that this temperature is somewhat lower than those (typically −20°C) at which full-core profiling methods are collected (e.g. ECM, DEP). Cole–Cole plumbing fixtures (Table 2) separates the private dielectric relaxations that combine to produce the observed dielectric spectra, and Figure 2 shows representative examples of these fits. These multiple relaxations include both the polarization of protonic defects in the ice lattice and extraneous electrode polarizations (labeled as 'other' in Fig. 2). F-tests point that fitting of multiple relaxations is highly significant (p < 0.01) because the normalized reduction in χ two typically exceeds 95% and the number of parameters added for each relaxation (3) is minor compared to the number of spectral data points (∼50 complex values).
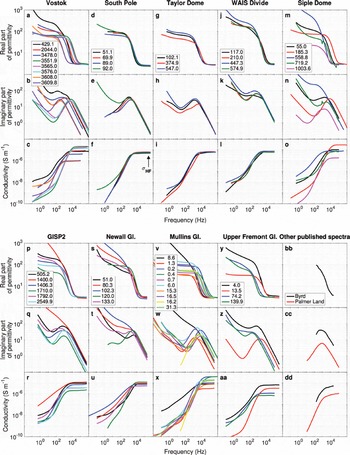
Fig. 1. Dielectric spectra of all samples at −forty°C. Each cavalcade represents a different ice core, and the private curves represent each sample (legend gives depth (m)). The meridian two rows for each sample are the complimentary-space normalized real ε′ and imaginary ε″ parts of the circuitous relative permittivity (i.eastward. the real and imaginary dielectric constants). The bottom row shows real electrical conductivity . Dielectric relaxations can be identified as peaks in ε″ and inflections in ε′ . Note high-frequency (HF) conductivity at 300 kHz (σ HF) is highlighted for Due south Pole samples. Byrd and Palmer Country samples shown in lower correct column were digitized from measurements by Paren and Glen (1978) and Reynolds (1985), respectively.

Fig. 2. Cole–Cole fits at −xl°C for selected samples. Dielectric relaxations associated with the water ice lattice are shown in red and blueish. The d.c. conductivity and electrode polarizations are shown in grey (labeled as 'other'). RMS misfits are ∼one% and F-tests point that multiple relaxations significantly improve the fits.
Tabular array 2. Cole–Cole parameters
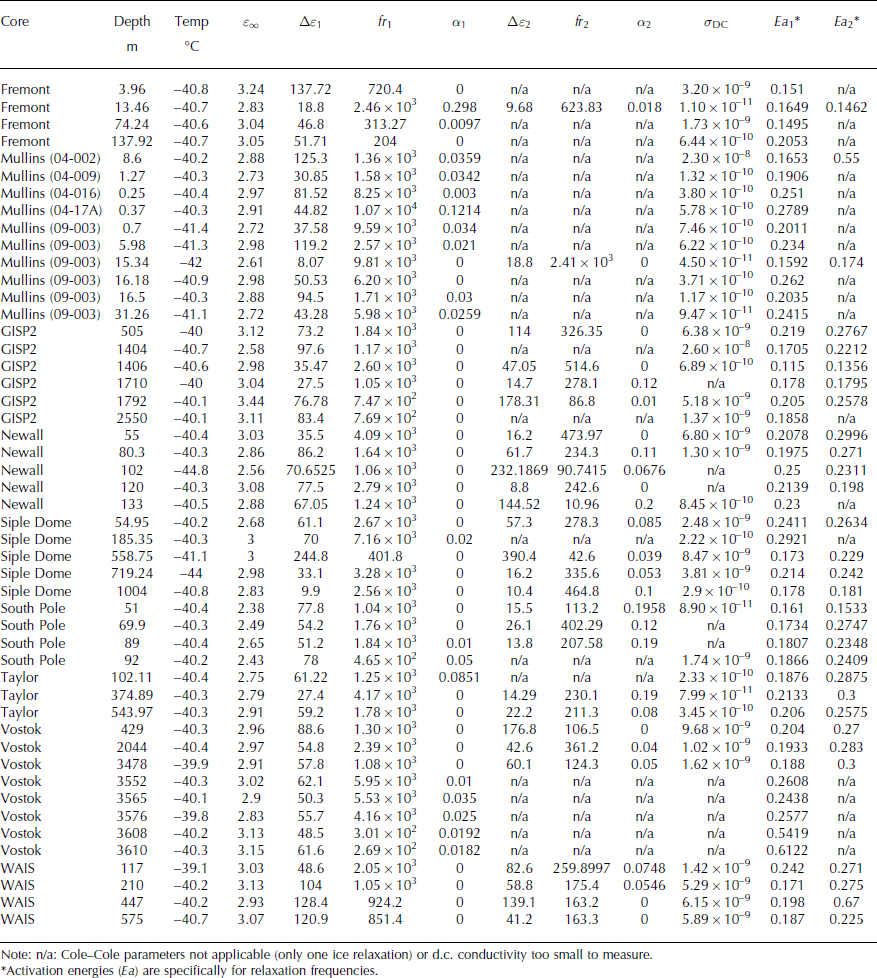
Figure 3 is an Arrhenius plot of relaxation frequency every bit a function of temperature, showing that individual relaxations associated with the ice lattice are consistently distinguishable in terms of both their magnitude and temperature dependence. Finally, Figure four shows modeled σ HF at −40°C for all samples and for each relaxation. We also utilize Jaccard theory to infer the concentration of soluble impurities in the lattice from f r (Appendix). Because we do not take complete bulk chemical science information for all samples, nosotros express the impurity concentration every bit the credible concentration of a unmarried species, alternatively [H+] or [Cl−] (Fig. 4b).

Fig. 3. Arrhenius plot of reciprocal temperature vs relaxation frequency for the samples shown in Figure two. The relaxation frequencies of water ice crystal populations are higher than those of laboratory-frozen pure ice (Kawada, 1978), whose change in slope is due to laboratory purity limit. These relaxations are likewise generally below Cl−-saturation limit (Grimm and others, 2008), except where NH4 +-enhanced Cl− sectionalisation is nowadays (Stillman and others, 2013b).

Fig. 4. (a) HF electrical conductivity of all samples at −40°C. (b) Credible lattice impurity concentrations, derived assuming either [H+] (left axis) or [Cl−] (right axis) was the only soluble impurity (Appendix). HF conductivities for two relaxations sometimes merge at -40°C, but fitting time constants at multiple temperatures (Fig. 3) assures that dual relaxations are resolved. Firn (open symbols) is distinguished from glacier ice (filled symbols). Two relaxations are common, even in firn, indicating that impurities are bimodally partitioned into crystal lattices during germination. Single relaxations in Vostok accreted water ice are the outcome of direct freezing from liquid. Unmarried relaxations of temperate ice trend toward increasing purity with depth, indicating that near-complete impurity ejection from the lattice is the end consequence of annealing.
A big majority (23/26) of the meteoric ice-sheet samples possess ii dielectric relaxations. A third relaxation was resolved in GISP2 (Greenland) 1406 m that has been discarded for clarity in the assay hither (Reference Stillman, MacGregor and GrimmStillman and others, 2013a). Two relaxations require two distinct impurity populations. Often, one of these relaxations has the characteristics of most pure water ice. The residuum (3/26) of the meteoric water ice-sheet samples take a unmarried relaxation. All of these samples are associated with loftier bulk impurity concentrations, although other samples with high impurity concentrations may still retain two relaxations. Vostok samples possess three distinct behaviors: two relaxations associated with meteoric water ice (the three shallowest samples); a unmarried relaxation indicating high impurities from the impurity-rich blazon i accreted ice; and a single relaxation indicating relatively high purity in the deeper lake-accreted ice (type two; the two deepest samples).
Due to the presence of insoluble inclusions, the first blazon (1) of Vostok accretion ice is believed to accept frozen onto either the grounded base of the E Antarctic ice sheet upstream of Vostok Subglacial Lake itself or within a shallow bay upstream of the main lake body (Reference Bong, Studinger, Tikku, Clarke, Gutner and MeertensBell and others, 2002; Reference De Angelis, Petit, Savarino, Souchez and ThiemensDe Angelis and others, 2004; Reference MacGregor, Matsuoka and StudingerMacGregor and others, 2009). Its relatively high σ HF values are equivalent to apparent [Cl−] ∼10−iv Thousand, or a source-water concentration ∼0.1 1000 assuming g = ten−three. This outcome is consistent with Cl− as the major impurity in this ice (Reference De Angelis, Petit, Savarino, Souchez and ThiemensDe Angelis and others, 2004) and a brackish source. At the other farthermost, the lake-accreted ice (blazon 2; 3608 and 3610 chiliad) is nearly pure, as to be expected from a very low partition coefficient and a big freshwater body (Reference Kapitsa, Ridley, Robin, Siegert and ZotikovKapitsa and others, 1996).
The sample from Mullins Glacier nearest the accumulation zone has 2 relaxations, and, as with the ice sheets, one indicates a population of nigh pure water ice. The other Mullins Glacier samples have a unmarried relaxation, with the notable exception of a sample near the finish of the active portion of the glacier whose two relaxations bespeak two distinct high-impurity populations. Many of the Mullins Glacier samples have among the highest HF conductivities and apparent soluble lattice impurities in our written report. Upper Fremont Glacier samples mostly have unmarried relaxations, and its deepest sample (>90% of the local ice thickness) indicates a virtually-pure ice lattice. Firn samples (6 total) have either one (2/6) or 2 (4/6) relaxations and thus are similar to densified water ice in terms of Cole–Cole behavior.
Word
Chief features of dielectric signatures
Both the characteristic relaxation frequencies and their associated activation energies observed in many of our samples autumn within ranges expected for protonic point defects in water ice (Fig. iii). Therefore, nosotros ascribe dual relaxations to the ice lattice, rather than attributing ane to another mechanism such every bit a Maxwell–Wagner interfacial polarization. Low-frequency (<one Hz at −xl°C; Fig. two) relaxations in our measurements, exterior the ice-lattice range, tin be attributed to electrode polarization. Boosted polarizations take been reported in shaved glacier ice (Reference Paren and GlenParen and Glen, 1978) and artificially dispersed ice (Reference Boned, Lagourette and ClausseBoned and others, 1979). These polarizations also lie outside the protonic-defect domain, and we advise they are interfacial effects. For water ice grown from liquid in the laboratory, we take never observed two or more relaxations that tin be attributed to the lattice (cf. Reference Von Hippel, Knoll and Westphalvon Hippel and others, 1971, et seq.). At very loftier salt concentrations in the parent liquid (>1 Yard), the dielectric relaxation of table salt hydrate tin appear (Reference Grimm, Stillman, Dec and BullockGrimm and others, 2008), but this complication is not relevant to low-impurity glacier ice.
The presence of two relaxations, therefore, appears to be unique to meteoric ice and indicates two separate ices with different impurity concentrations. If these impurities were well mixed, they would yield a single relaxation whose frequency was related to the mean impurity concentration, just peradventure with a broader distribution of length scales of charge motion (larger α). These two impurity populations could represent two distinct crystal populations, bimodal zonation within crystals, or a large number of separate, smaller bimodal electrical domains inside a crystal. The last possibility is especially speculative, but could involve enhanced concentrations of defects virtually dislocation cores necessary to overcome the proton-disorder barrier, or screening charge clouds that surround dislocation dangling bonds (Reference Petrenko and WhitworthPetrenko and Whitworth, 1999).
Impurity segmentation into the ice lattice could exist afflicted by the nature of impurity deposition on the subaerial surface (Reference Stillman, MacGregor and GrimmStillman and others, 2013a), i.due east. whether it occurs through droplets degradation ('dry') or whether impurities are incorporated within snowflakes ('wet'). Information technology is non articulate how wet vs dry impurity degradation could generate distinct grain populations. On the other mitt, scanning-electron and Ten-ray imaging of water ice (Reference Mulvaney, Wolff and OatesMulvaney and others, 1988; Reference Barnes, Wolff, Mallard and MaderBarnes and others, 2003) revealed that NaCl and other salts are present equally inclusions within ice grains. The threshold concentration for impurity detection with these methods (∼ten−3 Yard) greatly exceeds bulk concentrations (∼10−6 M). Therefore, the lattice around the inclusions may be significantly enhanced in soluble ions as sensed dielectrically, forming multiple 'cores' of impurities surrounded past a 'mantle' of relatively pure ice.
Alternatively, lattice impurities may be pushed toward grain boundaries immediately upon recrystallization from snowfall to firn. This arrangement is supported by microscopic imaging (Reference Barnes, Wolff, Mallard and MaderBarnes and others, 2003) that identified coating of grain boundaries with salts and acids. Again, the high detection threshold for those imaging methods suggests that a zone of dielectrically impure ice may be next to such grain-boundary coatings. In this configuration, a single purer core of each grain is surrounded by an impure mantle.
The lower bound for the dimension of electrical domains is the characteristic polarization deportation of protonic-point defects, which we estimate to be ∼one μm (encounter Appendix). If zonation existed on a smaller scale, charges would be unable to move through a sinusoidal cycle at the relaxation frequency and the relaxation spectrum would be distorted. Considering this dimension is a very minor fraction of the typical grain bore, the cadre/drapery zonation hypothesis posed above is tractable. However, dislocation widths may be as small as 1 unit prison cell, so associated point-defect zones might exist tens of unit cells wide, i.due east. ∼0.01 μm. Hence, nosotros conclude that private dislocations are too small to be the sources of the bimodal dielectric relaxations observed in glacier ice. All the same, dislocations are known to collaborate over longer distances (Reference Duval, Montagnat, Grennerat, Weiss, Meyssonnier and PhilipDuval and others, 2010), so an consequence of dislocations over larger scales cannot be ruled out.
Single-relaxation ice represents the minority of our samples, just information technology is not rare and occurs in all ice types we examined. Three of the meteoric water ice-sheet samples (Siple Dome (Antarctica) 185 m; GISP2 2250 k; Newall Glacier (Antarctica) 133 1000) possess single relaxations and were amongst the well-nigh impure samples for those corresponding cores that we sampled (Reference Stillman, MacGregor and GrimmStillman and others, 2013a). Single relaxations boss the Vostok accreted ice (5/5) and both tall glaciers (ten/12). The Mullins Glacier samples have σ HF values that are college than average, which presumably reflects the college concentration of lattice-soluble impurities that accompanies the source-zone debris input. Even so, there is no articulate relationship between dielectric signature and macroscopic englacial debris concentration or even water ice discoloration in Mullins Glacier samples (Tabular array 1). Considering discolored ice is establish at many levels in the cores, information technology is probable an effect of variable dust loading and not basal entrainment (cf. Reference Holdsworth and BullHoldsworth and Balderdash, 1970). Farther, the concentration of englacial droppings even in discolored samples of Mullins Glacier must be modest, because no ice–silicate interfacial polarizations are evident (Reference OlhoeftOlhoeft, 1977; Reference Stillman, Grimm and DecStillman and others, 2010; Reference Grimm and StillmanGrimm and Stillman, 2015).
Evolution of dielectric signatures
There are several key observations that shed light on the dielectric development of glacier ice. (1) Two relaxations are widespread in ice and firn. In GISP2, 2 relaxations are preserved through normal recrystallization/grain growth. In many water ice sheets, ii relaxations are however evident at the greatest ages sampled, up to a few hundred one thousand years. (two) Conversely, the iii ice-sheet samples that accept simply one relaxation are relatively impure, or are the deepest/ oldest in that core. Mullins Glacier samples, dominated past unmarried relaxations (six/eight), are both impure and old (at to the lowest degree several hundred thousand years in the analyzed core; notation that the putative 8 Ma ice is at the glacier terminus). (3) In temperate Upper Fremont Glacier, iii/4 of the samples take just one relaxation, and the iii single relaxations trend toward lower σ HF with increasing depth. We at present consider the significance of these observations for understanding the development of soluble impurities within glacier water ice. Table 3 summarizes the relationships between observations and our proposed development.
Table 3. Summary of types of dielectric signatures and associated glacier ice types. Meteoric ice evolves from upper left to lower right

We hypothesize that annealing, probably grain internal recovery, can explain part or all of these observations. We adopt the metallurgical definition of annealing (Reference DohertyDoherty and others, 1997) every bit the sum of processes that cause high-angle grain-boundary migration (recrystallization) and those internal processes removing dislocations and point defects (recovery). Because firn can besides have 2 relaxations, nosotros assume that all meteoric ice initially possesses (or is at least capable of possessing) two relaxations (dielectric populations). As ice ages, annealing causes these 2 populations to merge into one, and this procedure is strongly temperature-dependent. Anomalously high concentrations of lattice-soluble impurities accelerate annealing and hence the population-merging process. Annealing further purifies ice by progressively removing lattice defects.
In terms of the specific hypotheses introduced to a higher place for the nature of the dielectric domains, the annealing hypothesis does not favor ii initially distinct crystal populations, considering merging during normal recrystallization would and so immediately combine the 2 dielectric relaxations into one. Even so, multiple impure cores formed around droplets nuclei could exist preserved while grain boundaries are changing, or impure mantles could continuously remerge on migrating grain boundaries.
Preservation of two dielectric relaxations through the process of normal grain recrystallization implies further that the subsequent process that merges dielectric domains is grain internal recovery. Specifically, grain growth in GISP2 is complete in <5 ka (Reference GowGow and others, 1997; Reference MeeseMeese and others, 1997), notwithstanding two dielectric relaxations are evident for at least 20 ka. Indeed, several of our samples have ages exceeding 100 ka and still evidence two dielectric relaxations (Vostok 2044 m, Taylor Dome (Antarctica) 544 m and Mullins Glacier MCI-09-003 15.iii chiliad; Tabular array 1).
Figure 4 shows that at that place is no clear relationship between dielectric signature and mean annual surface temperature (a elementary proxy for depositional weather condition) and but hints at such a relationship with depth (a simple proxy for fourth dimension). I of these three samples is ∼150 ka sometime (Vostok 2044 m), implying that over this period at low in situ temperatures (<−35°C), it has non still experienced sufficient annealing to merge the impurity populations. Our deepest GISP2 sample (2550 m) had only a unmarried relaxation and is only 67 ka onetime, but it experienced generally higher temperatures. We notation that typical surface speeds of the Antarctic ice sheet are ∼10 ma−one (Reference Rignot, Mouginot and ScheuchlRignot and others, 2011) and all of the ice-core samples nosotros measured were fatigued from average- to slower-flowing locations. Samples from fast-moving ice (>100 thou a−one) would assist farther constrain the relative importance of cumulative strain upon dielectric evolution.
These slow rates for merging dielectric populations imply lower diffusion coefficients D or higher activation energies E a than those derived for recrystallization. For instance, Due east a for volume self-improvidence (62 kJ mol−i; Reference Petrenko and WhitworthPetrenko and Whitworth, 1999) is larger than that for recrystallization (42 kJ mol−1; Reference Cuffey and PatersonCuffey and Paterson, 2010). However, even when accounting for these possible differences, D must be at least 2 orders of magnitude smaller than the volume self-diffusion coefficient (Reference Petrenko and WhitworthPetrenko and Whitworth, 1999). Assuming t > t d ∼ r ii/π 2 D, where t is the observed greatest historic period with two relaxations, t d is a feature diffusion time and r is the grain radius ( 3 mm), nosotros find that D must exist less than ∼10−18 −10−17 mii s−1 at −twenty°C to account for the long recovery times implied by our observations of the Siple Dome, Byrd, GISP2, Taylor Dome and Vostok ice cores. We hypothesize that the Mullins Glacier sample from the ∼600 ka core (MCI-09-003) that nevertheless possesses 2 relaxations was further delayed in annealing due to insoluble-impurity pinning or an anomalously low temperature.
The common blueprint of two relaxations with variable degrees of apparent purity is consistent with a stochastic climatic overprint associated with variable impurity deposition and the well-recognized dielectric stratigraphy of ice cores recorded past ECM and DEP. This overprint tin can occasionally produce an anomalous dielectric signature (i.e. a single relaxation), despite similar concrete conditions and history (temperature and age), equally appears to have occurred for at least three of our samples (Siple Dome 185 1000; GISP2 2250 yard; Newall Glacier 133 m). Based on the dielectric signatures of these samples, nosotros further hypothesize that unusually high concentrations of lattice-soluble impurities accelerate annealing.
In dissimilarity, ice with the oldest double relaxation observed in temperate Upper Fremont Glacier was formed simply about a decade before coring, suggesting that D is only a few times smaller than expected for volume diffusion. Reference Reynolds and ParenReynolds and Paren (1980) invoked faster recrystallization rates in temperate glaciers, as compared to polar glaciers, to explain the lower d.c. conductivity of the erstwhile. Yet, their hypothesis has been invalidated past the discovery that 'd.c.' polar field measurements were instead generally sensitive to the HF response of ice (Reference Stillman, MacGregor and GrimmStillman and others, 2013a). However, annealing remains fundamental to our present hypothesis every bit to why temperate glaciers rapidly evolve dielectrically into a single-relaxation material.
Ultimately, soluble impurities may be completely ejected from the lattice to the intergranular surfaces, where they no longer have a dielectric relaxation because they are in intergranular liquid or take become enclathrated. In Upper Fremont Glacier, the iii samples showing single relaxations are increasingly pure with depth. Meltwater flushing cannot directly touch lattice-incorporated impurities, so either this ordering is coincidence (17% iii-way permutation probability) or lattice impurity concentrations are decreasing with increasing depth or time. The time to evolve toward near pure water ice appears to be a few centuries, also consistent with book improvidence. The lower temperatures, higher activation energies, lower improvidence coefficients or higher proportion of insoluble impurities appropriate to non-temperate water ice inhibit this progression.
Single relaxations with loftier Cl− concentrations are as well institute in Vostok accreted ice type 1. Although the single relaxation is readily distinguished as a primary difference betwixt accreted or laboratory-frozen ice compared to meteoric water ice, the apparent lack of annealing toward a pure lattice is unexpected. Larger improvidence times would be associated with the larger (∼20cm) crystals of Vostok accreted ice blazon 1; therefore, if impurity rejection is controlled in the aforementioned way equally Upper Fremont Glacier, the upper limit to the age of this accreted ice is ∼grand years. The high purity of accreted ice blazon ii likely arises directly from partitioning from Vostok Subglacial Lake.
Conclusions
We surveyed the broadband dielectric behavior (0.1 Hz–1 MHz) of 49 samples of naturally formed glacier ice (including ii from previous studies), produced by multiple formation mechanisms, containing varying impurity concentrations and subjected to differing temperature histories. This large sample size enabled a showtime qualitative nomenclature and analysis of the variety of broadband dielectric signatures that are observed in such ice. A synthesis of these measurements reveals the following: (ane) Meteoric polar ice more often than not possesses ii dielectric relaxations representing a bimodal zonation of impurity domains. These zones probable either environment insoluble impurities within the lattice or form coatings near grain boundaries. (two) Higher temperatures, longer time periods and higher impurity concentrations all tend to consequence in a single-relaxation dielectric signature, presumably reflecting greater cumulative annealing, specifically grain internal recovery. Not all samples obey these relationships, further indicating the importance of spatio-temporally variable impurity deposition in modulating the dielectric signature of meteoric ice. (3) Subglacially accreted (lake-frozen) ice possesses only one relaxation, whose caste of purity likely reflects the accretion charge per unit and composition of the source water.
These common dielectric signatures underlie the time-variable impurity degradation that produces the variable bulk signals typically observed in ECM and DEP logs. A fundamental source of uncertainty in our conclusions that warrants further investigation is a quantitative evaluation of the relationship between annealing rates and dielectric backdrop (east.thou. HF conductivity). In particular, the slower diffusion implied by the dielectric evolution compared to volume improvidence requires further investigation. Nosotros suggest that focused application of other tools practical to written report ice (eastward.g. electron microscopy, diffraction topography, measurements at radio- to infrared frequencies) may reveal the origin of singled-out impurity domains.
This report further demonstrates the utility of broadband (<1 Hz to >100 kHz) laboratory measurements of circuitous permittivity for understanding the origin and evolution of glacier ice using cut core samples. Multi-frequency profiling of full cores would provide spatially consummate cadre information. We recommend that the minimum frequency be reduced to <i Hz to allow robust Cole–Cole plumbing fixtures and separation of the constituent conduction and polarization mechanisms. Such a change would, however, decrease the speed of data acquisition to ∼one mm s−1. We too recommend that such full-core profiling be performed at the everyman temperature possible, well below the typical −20°C, every bit lower temperatures enhance separation and modeling of multiple ice relaxations.
Acknowledgements
This piece of work was funded by NASA'south Planetary Geology and Geophysics program (NNX14AN30G) and by Southwest Research Institute (Internal Enquiry Grant R8422). We acknowledge Mark Twickler, Geoffrey Hargreaves and Richard Nunn for admission to and preparation of ice samples at the United states National Water ice Core Laboratory. We are grateful to Doug Kowalewski for discussions about Mullins Glacier and for initiating our sample requests. We thank Steve Arcone and an bearding reviewer for constructive comments.
Appendix
Jaccard theory and apparent soluble-impurity concentrations
We use Jaccard theory (Reference Petrenko and WhitworthPetrenko and Whitworth, 1999) to make up one's mind the concentration of lattice defects from the measured dielectric properties (Fig. four). We express these defect concentrations alternatively as the credible lattice concentration of H+ or Cl−, because these impurities are dominant in naturally formed ice. We announce these concentrations equally 'apparent' because either, both, or their combination with NHfour + could produce the observed dielectric signature. These concentrations were estimated using a dimensionless constant G = 3, the mean distance between oxygen ions in the water ice lattice (rOO = two.764 ×10−10 m), the mobility of Fifty-defects at ![]() , the mobility of ionic defects at
, the mobility of ionic defects at ![]() , the accuse of Fifty-defects (e 50 = 0.38e, where e is the charge of a proton) and the accuse of ionic defects (e + = 0.62e). The temperature dependence of was analyzed to guess [Cl−] and the activation energy of the mobility. We also assume that a unmarried Cl− ion produces two L-defects, i.e. north L = 2[Cl−], while a single H+ ion produces a single ionic defect n + = [H+]. Jaccard theory defines and as
, the accuse of Fifty-defects (e 50 = 0.38e, where e is the charge of a proton) and the accuse of ionic defects (e + = 0.62e). The temperature dependence of was analyzed to guess [Cl−] and the activation energy of the mobility. We also assume that a unmarried Cl− ion produces two L-defects, i.e. north L = 2[Cl−], while a single H+ ion produces a single ionic defect n + = [H+]. Jaccard theory defines and as
(A1)
![]()
(A2)
![]()
(A3)
![]()
where ![]() , 1000 B is the Boltzmann constant and T is temperature. We then assume alternately that the relaxation is due to ionic or L-defects just:
, 1000 B is the Boltzmann constant and T is temperature. We then assume alternately that the relaxation is due to ionic or L-defects just:
(A4)
![]()
The end-member conductivities in Eqn (A4) tin can then exist translated to end-member (apparent) concentrations start by assuming that 50-defects dominate and fitting observed τ as a function of temperature to find the activation energy of μ L.
(A5)
![]()
Once the apparent [Cl−] has been calculated, apparent [H+] follows from
(A6)
![]()
(A7)
![]()
(A8)
![]()
(A9)
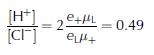
Length calibration of charge motility
Nosotros exploit the illustration between catamenia of rut and accuse to determine the characteristic length calibration of protonic-defect deportation inside the ice lattice in response to an applied electric field. The e-folding time constant is derived from the polarization response to a step modify in voltage. Following a footstep change in temperature to a ane-dimensional half-space, the corresponding length scale as a function of time is approximately ![]() , where D is the diffusion coefficient (Reference Carslaw and JaegerCarslaw and Jaeger, 1959). The angular relaxation frequency is the reciprocal of the relaxation time. We take τ = eight.4 × 10−14 exp(5000/T) every bit representative of our samples; this function is logarithmically intermediate betwixt pure and chloride-saturated water ice.
, where D is the diffusion coefficient (Reference Carslaw and JaegerCarslaw and Jaeger, 1959). The angular relaxation frequency is the reciprocal of the relaxation time. We take τ = eight.4 × 10−14 exp(5000/T) every bit representative of our samples; this function is logarithmically intermediate betwixt pure and chloride-saturated water ice.
We and so calculate D = chiliad B Tμ Fifty/e L (Reference Petrenko and WhitworthPetrenko and Whitworth, 1999), where the mobility and accuse are appropriate to the bulk L-defects, which dominate a.c. conduction. Using the values given to a higher place for μ L and e L, we summate δ = 0.5 μm at −20°C. The activation energy 0.24 eV for L-defects increases δ only to 0.9 μm at −fifty°C. Therefore, the feature length calibration to which defect charges can be dielectrically displaced is large compared to the lattice spacing (0.three nm), but small compared to typical grain sizes (>one mm). We note that there are no theoretical limitations to accuse movement under a steady (d.c.) applied electric current, where lattice conductivity is determined by the appropriate balance of defect types (Reference Petrenko and WhitworthPetrenko and Whitworth, 1999) modulated by grain-purlieus resistance (Stillman and others, 2010).
References
Barnes, PRF , Wolff, EW , Mallard, DC and Mader, HM (2003) SEM studies of the morphology and chemistry of polar water ice. Microsc. Res. Tech., 62(i), 62–69 CrossRefGoogle ScholarPubMed
Barsukov, E and Macdonald, JR (2005) Impedance spectroscopy: theory, experiment, and applications. Wiley, Hoboken, NJ CrossRefGoogle Scholar
Bell, RE , Studinger, M , Tikku, AA , Clarke, GKC , Gutner, MM and Meertens, C (2002) Origin and fate of Lake Vostok water frozen to the base of the East Antarctic ice sheet. Nature, 416(6878), 307–310 CrossRefGoogle ScholarPubMed
Blunier, T and Brook, EJ (2001) Timing of millennial-calibration climate change in Antarctica and Greenland during the terminal glacial period. Scientific discipline, 291, 109–112 CrossRefGoogle ScholarPubMed
Bohleber, P , Wagner, N and Eisen, O (2012) Permittivity of water ice at radio frequencies: Part I. Coaxial transmission line cell. Cold Reg. Sci. Technol., 82, 56–67 (doi: 10.1016/j.coldregions.2012.05.011)CrossRefGoogle Scholar
Boned, C , Lagourette, B and Clausse, M (1979) Dielectric behaviour of water ice microcrystals: a study versus temperature. J. Glaciol., 22(86), 145–154 CrossRefGoogle Scholar
Beck, EJ and 8 others (2005) Timing of millennial-calibration climate change at Siple Dome, West Antarctica, during the last glacial period. Quat. Sci. Rev., 24, 1333–1343 (doi: x.1016/ j.quascirev.2005.02.002)CrossRefGoogle Scholar
Camplin, GC , Glen, JW and Paren, JG (1978) Theoretical models for interpreting the electrical behaviour of HF-doped ice. J. Glaciol., 21(85), 123–141 CrossRefGoogle Scholar
Carslaw, HS and Jaeger, JC (1959) Conduction of rut in solids. Oxford University Press, Oxford Google Scholar
Cole, KS and Cole, RH (1941) Dispersion and adsorption in dielectrics. I: Alternate current characteristics. J. Chem. Phys. nine(four), 341–352 CrossRefGoogle Scholar
Cuffey, KM and Paterson, WSB (2010) The physics of glaciers, 4th edn. Elsevier, Amsterdam Google Scholar
De Angelis, G , Petit, JR , Savarino, J , Souchez, R and Thiemens, MH (2004) Contributions of an ancient evaporitic-type reservoir to subglacial Lake Vostok chemistry. Globe Planet. Sci. Lett., 222, 751–765 (doi: ten.1016/j.epsl.2004.03.023)CrossRefGoogle Scholar
Debye, PJW (1929) Polar molecules. Chemic Catalog Company, New York Google Scholar
Delmas, RJ , Kirchner, Due south , Palais, JM and Petit, JR (1992) 1000 years of explosive volcanism recorded at the Southward Pole. Tellus B, 44, 335–350 CrossRefGoogle Scholar
Doherty, RD and nine others (1997) Current issues in recrystallization: a review. Mat. Sci. Eng., A238, 219–274 CrossRefGoogle Scholar
Dowdeswell, JA and Evans, S (2004) Investigations of the grade and flow of ice sheets and glaciers using radio-repeat sounding. Rep. Progr. Phys., 67, 1821–1861 (doi: 1088/0034-4885/ 67/10/R03)CrossRefGoogle Scholar
Duval, P , Montagnat, M , Grennerat, F , Weiss, J , Meyssonnier, J and Philip, A (2010) Creep and plasticity of glacier water ice: a cloth scientific discipline perspective. J. Glaciol., 56(200), 1059–1068 (doi: 10.3189/002214311796406185)CrossRefGoogle Scholar
Eisen, O , Wilhelms, F , Steinhage, D and Schwander, J (2006) Improved method to determine radio-echo sounding reflector depths from ice-cadre profiles of permittivity and conductivity. J. Glaciol., 52(177), 299–310 (doi: 10.3189/172756506781828674)CrossRefGoogle Scholar
Fitzgerald, WJ and Paren, JG (1975) The dielectric properties of Antarctic ice. J. Glaciol. 15(73), 39–59 CrossRefGoogle Scholar
Fujita, S , Matsuoka, T , Ishida, T , Matsuoka, Chiliad and Mae, South (2000) A summary of the complex dielectric permittivity of ice in the megahertz range and its applications for radar sounding of polar water ice sheets. In Hondoh, T ed. Physics of ice cadre records. Hokkaido Academy Press, Sapporo, 185–212 Google Scholar
Glen, JW , Homer, DR and Paren, JG (1977) Water at grain boundaries: its role in the purification of temperate glacier water ice. IAHS Publ. 118 (Symposium at Grenoble 1975 – Isotopes and Impurities in Snow and Water ice), 263–271 Google Scholar
Gow, AJ and 6 others (1997) Physical and structural properties of the Greenland Ice Canvass Project 2 water ice cadre: a review. J. Geophys. Res., 102(C12), 26 559–26 575 CrossRefGoogle Scholar
Grimm, RE and Stillman, DE (2015) Field examination of detection and characterization of subsurface ice using broadband spectral induced polarization. Permafrost Periglac. Procedure., 26, 28–38 (doi: 10.1002/ppp.1833)CrossRefGoogle Scholar
Grimm, RE , Stillman, DE , Dec, SF and Bullock, MW (2008) Depression-frequency electrical backdrop of polycrystalline saline ice and table salt hydrates. J. Phys. Chem. B, 112(48), fifteen 382–fifteen 390 (doi: 10.1021/jp8055366)CrossRefGoogle ScholarPubMed
Gross, GW , Wong, PM and Humes, Thou (1977) Concentration dependent solute redistribution at the ice–water phase boundary. III. Spontaneous convection. Chloride solutions. J. Chem. Phys., 67(11), 5264–5274 CrossRefGoogle Scholar
Hammer, CU (1980) Acerbity of polar water ice cores in relation to accented dating, by volcanism, and radio-echoes. J. Glaciol., 25(93), 359–372 CrossRefGoogle Scholar
Holdsworth, G and Bull, C (1970) The flow law of cold ice: investigations on Meserve Glacier, Antarctica. IAHS Publ. 86 (Symposium at Hanover 1968 – Antarctic Glaciological Exploration (ISAGE)), 204–216 Google Scholar
Jaccard, C (1959) Thermodynamics of irreversible processes practical to ice. Phys. Kondens. Mater., three, 99–118 Google Scholar
Jouzel, J and 9 others (1999) More than than 200 meters of lake water ice to a higher place subglacial Lake Vostok. Scientific discipline, 286, 2138–2141 CrossRefGoogle ScholarPubMed
Kapitsa, AP , Ridley, JK , Robin, GdeQ , Siegert, MJ and Zotikov, IA (1996) A large deep freshwater lake beneath the ice of the key East Antarctic. Nature, 381(6584)CrossRefGoogle Scholar
Kulessa, B (2007) A critical review of the low-frequency electric backdrop of ice sheets and glaciers. J. Environ. Eng. Geophys. 12(ane), 23–36 CrossRefGoogle Scholar
MacGregor, JA , Matsuoka, G and Studinger, One thousand (2009) Radar detection of accreted ice over Lake Vostok, Antarctica. Globe Planet. Sci. Lett., 282, 222–233 (doi: x.1016/j.epsl.2009.03.018)CrossRefGoogle Scholar
MacGregor, JA and 11 others (2015) Radar attenuation and temperature within the Greenland Ice Sheet. J. Geophys. Res. Earth Surf., 120 (doi: 10.1002/2014JF003418)CrossRefGoogle Scholar
Maeno, N (1978) The electrical behaviours of Antarctic ice drilled at Mizuho Station, East Antarctica. Mem. Natl Inst. Polar Res., 10, 77–94 Google Scholar
Marchant, DR and 8 others (2007) Establishing a chronology for the world's oldest glacier ice. USGS Open-File Rep. 2007-1047, 054Google Scholar
Mayewski, PA and eleven others (1995) An ice-core-based, late Holocene history for the Transantarctic Mountains, Antarctica. Antarct. Res. Ser. 67, 33–45 CrossRefGoogle Scholar
Mayewski, PA and 13 others (1996) Climatic change during the last deglaciation in Antarctica. Science, 272, 1636–1638 CrossRefGoogle ScholarPubMed
Meese, DA and 8 others (1997) The Greenland Ice Sheet Project 2 depth–age scale: methods and results. J. Geophys. Res., 102(C2), 26 411–26 423 CrossRefGoogle Scholar
Moore, JC and Paren, JG (1987) A new technique for dielectric logging of Antarctic ice cores. J. Phys. [Paris], 48(C1), 155–160 Google Scholar
Mulvaney, R , Wolff, EW and Oates, K (1988) Sulphuric acid at grain boundaries in Antarctic ice. Nature, 331(6153), 247–249 CrossRefGoogle Scholar
Olhoeft, GR (1977) Electric properties of natural clay permafrost. Tin can. J. Earth Sci., 14, 16–24 CrossRefGoogle Scholar
Paren, JG and Glen, JW (1978) Electrical behaviour of finely divided ice. J. Glaciol., 21(85), 173–191 CrossRefGoogle Scholar
Petit, JR and 18 others (1999) Climate and atmospheric history of the by 420,000 years from the Vostok ice core, Antarctica. Nature, 399, 429–436 CrossRefGoogle Scholar
Petrenko, VF and Whitworth, RW (1999) Physics of ice. Oxford University Press, Oxford Google Scholar
Reynolds, JM and Paren, JG (1980) Recrystallisation and the electrical behaviour of glacier ice. Nature, 283(5742), 63–64 CrossRefGoogle Scholar
Reynolds, M (1985) Dielectric behaviour of firn and water ice from the Antarctic Peninsula, Antarctica. J. Glaciol., 31(109), 253–262 CrossRefGoogle Scholar
Rignot, East , Mouginot, J and Scheuchl, B (2011) Ice flow of the Antarctic water ice sheet. Science 333(6048), 1427–1430 (doi: 10.1126/science.1208336)CrossRefGoogle ScholarPubMed
Schuster, PF , White, DE , Naftz, DL and Cecil, LD (2000) Chronological refinement of an ice cadre record at Upper Fremont Glacier in south central North America. J. Geophys. Res., 105, 4657–4666 (doi: 10.1029/1999JD901095)CrossRefGoogle Scholar
Stillman, DE , Grimm, RE and Dec, S (2010) Low-frequency electrical properties of ice–silicate mixtures. J. Phys. Chem. B, 114(18), 6065–6073 (doi: 10.1021/jp9070778)CrossRefGoogle ScholarPubMed
Stillman, DE , MacGregor, JA and Grimm, RE (2013a) The function of acids in electrical conduction through ice. J. Geophys. Res., 118 (doi: 10.1029/2012JF002603)Google Scholar
Stillman, DE , MacGregor, JA and Grimm, RE (2013b) Electrical response of ammonium-rich water ice. Ann. Glaciol., 54(64), 21–26 (doi: 10.3189/2013AoG64A204)CrossRefGoogle Scholar
Sugden, DE and half-dozen others (1995) Preservation of Miocene glacier ice in East Antarctica. Nature, 376, 412–414 CrossRefGoogle Scholar
Sugiyama, K and 7 others (2000) Measurement of electric conductance in ice cores past AC-ECM method. In Hondoh, T ed. Physics of ice core records. Hokkaido University Press, Sapporo, 173–184 Google Scholar
Taylor, KC and seven others (1993) The 'flickering switch' of late Pleistocene climate change. Nature, 361, 432–436 CrossRefGoogle Scholar
Von Hippel, A , Knoll, DB and Westphal, WB (1971) Transfer of protons through 'pure' ice Ih single crystals. I. Polarization spectra of water ice Ih . J. Chem. Phys., 54(1), 134–144 CrossRefGoogle Scholar
WAIS Divide Project Members (2013) Onset of deglacial warming in Westward Antarctica driven by local orbital forcing. Nature, 500, 440–444 (doi: ten.1038/nature12376)CrossRef
Wilhelms, F , Kipfstuhl, J , Miller, H , Heinloth, K and Firestone, J (1998) Precise dielectric profiling of ice cores: a new device with improved guarding and its theory. J. Glaciol., 44(146), 171–174 CrossRefGoogle Scholar
Wolff, EW (2000) Electrical stratigraphy of polar ice cores: principles, methods and findings. In Hondoh, T ed. Physics of ice core records. Hokkaido University Press, Sapporo, 155–171 Google Scholar
Wolff, EW and Paren, JG (1984) A ii-phase model of electric conduction in polar ice sheets. J. Geophys. Res., 89(B11), 9433–9438 CrossRefGoogle Scholar
Wolff, EW , Miners, WD , Moore, JC and Paren, JG (1997) Factors controlling the electrical electrical conductivity of ice from the polar regions – a summary. J. Phys. Chem. B, 101(32), 6090–6094 CrossRefGoogle Scholar
Source: https://www.cambridge.org/core/journals/journal-of-glaciology/article/dielectric-signatures-and-evolution-of-glacier-ice/FD17C0C950E1226196122C7E59DE5456
0 Response to "What Happens to Snowflakes as They Become Progressively Buried Within Glaciers?"
Post a Comment